Ultomiris Drug Market: Driving Innovation in Rare Disease Treatment
The global pharmaceutical landscape has been witnessing significant advancements in recent years, particularly in the treatment of rare and ultra-rare diseases. One such breakthrough therapy that has gained attention is Ultomiris (ravulizumab-cwvz), developed by Alexion Pharmaceuticals (now part of AstraZeneca). As the first and only long-acting C5 complement inhibitor, Ultomiris represents a major shift in how physicians and patients approach chronic and life-threatening conditions such as paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and more recently, generalized myasthenia gravis (gMG). The Ultomiris drug market is expanding steadily, driven by rising demand for advanced biologics, increasing rare disease awareness, and strong R&D pipelines.
GET REPORT LINK:https://m2squareconsultancy.com/reports/ultomiris-drug-market
Market Overview
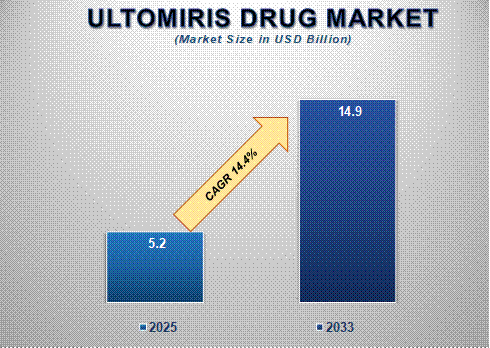
The global Ultomiris drug market size is poised for significant expansion, with its market size projected to grow from USD 5.2 billion in 2025 to USD 14.9 billion by 2033, reflecting a strong compound annual growth rate (CAGR) of 14.4% during the forecast period. The market’s rapid growth is driven by the increasing prevalence of these rare and life-threatening disorders, advancements in targeted therapies, and the drug's proven clinical efficacy in extending dosing intervals compared to previous treatments like Soliris.
The global Ultomiris market is witnessing high growth, primarily due to:
-
Rising prevalence of rare complement-mediated disorders
-
Shift toward long-acting therapies
-
Strategic pricing and reimbursement approvals
-
Expansion into new geographies and indications
According to industry analysts, Ultomiris is positioned to become a multi-billion-dollar therapy over the next few years, capturing market share from both Soliris and other competing therapies in the rare disease space.
Key Growth Drivers
1. Growing Rare Disease Awareness
Governments and healthcare organizations are investing heavily in rare disease awareness campaigns, patient registries, and diagnostic infrastructure. Early detection of diseases like PNH, aHUS, and gMG has contributed to higher diagnosis rates, thus expanding the potential patient pool for Ultomiris.
2. Strong Clinical Pipeline and Indication Expansion
Ultomiris has already secured approvals in the U.S., Europe, and several other regions for PNH, aHUS, and gMG. Clinical trials are ongoing for additional indications, including neuromyelitis optica spectrum disorder (NMOSD). Each new indication adds to the therapy’s revenue potential and strengthens its competitive positioning.
3. Patient-Centric Advantages
One of Ultomiris’ strongest differentiators is its extended dosing schedule. While Soliris required biweekly infusions, Ultomiris is administered every 8 weeks for adults, drastically reducing hospital visits and improving patient quality of life. This convenience factor makes it highly attractive for patients and healthcare providers alike.
4. Strategic Backing from AstraZeneca
Since Alexion’s acquisition by AstraZeneca in 2021, Ultomiris has benefited from the latter’s vast global distribution network, R&D capabilities, and financial resources. AstraZeneca’s presence in emerging markets has further supported Ultomiris’ expansion into regions where access to advanced biologics was previously limited.
REPORT SAMPLE LINK:https://m2squareconsultancy.com/request-sample/ultomiris-drug-market
BUY NOW:https://m2squareconsultancy.com/purchase/51
Market Challenges
Despite its strong performance, the Ultomiris drug market faces certain challenges:
-
High cost of therapy: Ultomiris remains an expensive treatment, which can limit patient access in regions with limited healthcare funding.
-
Competition from biosimilars: As patents for Soliris and related therapies expire, biosimilars are entering the market, potentially putting pricing pressure on Ultomiris.
-
Regulatory hurdles: Expanding into new indications and geographies requires lengthy clinical trials and regulatory approvals, which can delay market growth.
-
Dependence on narrow patient pool: As a therapy for rare diseases, the overall patient base remains small compared to mainstream pharmaceuticals.
TRENDING REPORT:
https://m2squareconsultancy.com/reports/wearable-medical-devices-market
https://m2squareconsultancy.com/reports/global-energy-drinks-market
https://m2squareconsultancy.com/reports/std-diagnostics-market
https://m2squareconsultancy.com/reports/oncology-drugs-market
https://m2squareconsultancy.com/reports/ultomiris-drug-market
https://m2squareconsultancy.com/reports/sustainable-pharmaceutical-packaging-market
https://m2squareconsultancy.com/reports/syringe-infusion-pumps-market
https://m2squareconsultancy.com/reports/local-anesthesia-drugs-market
https://m2squareconsultancy.com/reports/hospital-acquired-infections-diagnostics-market
https://m2squareconsultancy.com/reports/biopolymers-and-bioplastics-market
https://m2squareconsultancy.com/reports/solar-panel-operation-and-maintenance-market
https://m2squareconsultancy.com/reports/fruit-and-vegetable-pulp-market
https://m2squareconsultancy.com/reports/spiritual-and-wellness-products-market
https://m2squareconsultancy.com/reports/portable-ultrasound-devices-market
https://m2squareconsultancy.com/reports/fecal-immunochemical-diagnostic-test-market
https://m2squareconsultancy.com/reports/next-generation-sequencing-market
https://m2squareconsultancy.com/reports/surgical-operating-tables-market
https://m2squareconsultancy.com/reports/floor-cleaner-market
Regional Insights
-
North America remains the largest market for Ultomiris, driven by strong healthcare infrastructure, favorable reimbursement policies, and high awareness of rare diseases.
-
Europe is another key region, supported by early approvals from the European Medicines Agency (EMA) and growing adoption among clinicians.
-
Asia-Pacific is emerging as a high-growth market due to rising healthcare spending, government initiatives for rare disease treatments, and expanded AstraZeneca operations in markets like Japan, China, and South Korea.
-
Middle East and Latin America also present untapped potential, although limited awareness and high therapy costs continue to restrict widespread adoption.
Competitive Landscape
Ultomiris competes not only with its predecessor Soliris but also with newer complement inhibitors and biosimilars. Companies such as Apellis Pharmaceuticals (with pegcetacoplan) are introducing alternatives that target different parts of the complement system. This increasing competition emphasizes the importance of continuous innovation, strong patient support programs, and strategic pricing models for Ultomiris to retain its dominance.
Future Outlook
The future of the Ultomiris drug market looks promising, as the therapy continues to expand into new patient populations and geographies. Analysts expect Ultomiris to play a pivotal role in AstraZeneca’s rare disease portfolio, with sales projected to grow steadily through the next decade.
Key trends shaping the future include:
-
Expansion into pediatric populations for PNH and aHUS.
-
Introduction of subcutaneous formulations, which would further reduce patient burden.
-
Collaborations with diagnostic companies to accelerate rare disease detection.
-
Government incentives and orphan drug designations that support innovation and market exclusivity.
Conclusion
Ultomiris is redefining treatment standards in rare diseases by offering a long-acting, highly effective, and patient-friendly therapy. While challenges such as high costs and growing competition exist, the drug’s clinical advantages and AstraZeneca’s strategic global push position it for strong growth. As healthcare systems continue to prioritize rare disease treatment and patient-centric care, Ultomiris is set to remain a cornerstone therapy in the complement inhibition market for years to come.
About m2squareconsultancy :
We are a purpose-driven market research and consulting company passionate about turning data into direction. Founded in 2023, we bring together researchers, strategists, and data scientists who believe that intelligence isn’t just about numbers, it’s about insight that sparks progress.
We cater to a wide range of industries by delivering customized solutions, strategic insights, and innovative support that help organizations grow, adapt, and lead in their respective sectors. Here’s a brief overview of key industries we work with.
Contact Us:
Email: sales@m2squareconsultancy.com
Phone (IN): +91 80978 74280
Phone (US): +1 929 447 0100
More Report:
https://m2squareconsultancy.com/reports/organic-personal-care-products-market
https://m2squareconsultancy.com/reports/portable-power-station-market
https://m2squareconsultancy.com/reports/power-transformer-market
https://m2squareconsultancy.com/reports/renewable-energy-market
https://m2squareconsultancy.com/reports/artificial-sweeteners-market
https://m2squareconsultancy.com/reports/blister-packaging-market
https://m2squareconsultancy.com/reports/digital-therapeutics-market
https://m2squareconsultancy.com/reports/drug-discovery-outsourcing-market
https://m2squareconsultancy.com/reports/e-pharmacy-market
https://m2squareconsultancy.com/reports/hospital-acquired-infections-diagnostics-market
https://m2squareconsultancy.com/reports/edible-oil-market
https://m2squareconsultancy.com/reports/premium-chocolate-and-confectionery-market
https://m2squareconsultancy.com/reports/dehydrated-vegetables-market
https://m2squareconsultancy.com/reports/spiritual-and-wellness-products-market
https://m2squareconsultancy.com/reports/portable-ultrasound-devices-market
https://m2squareconsultancy.com/reports/ophthalmic-lenses-market